Research
Memories are our window into who we are. They evolve as we mature, sometimes fade as we age, and are too often vulnerable to rapid decay in psychiatric illnesses. While the process of memory formation and retention has often been reduced to fit simple models, we increasingly appreciate that the deep well of neural diversity present in the brain is key to understanding its functions and dysfunctions. Our lab is focused on uncovering the computational principles, circuit mechanisms and cognitive consequences of memory circuit function throughout the lifespan and its dysfunction in neuropsychiatric diseases. In order to tackle these questions our lab will use a combination of cutting-edge tools including in vivo two-photon calcium imaging and electrophysiology to monitor, optogenetic and chemogenetic methods to manipulate neural activity together with transgenic cell-type targeting and circuit tracing strategies to establish causal relationships between diverse neural subtypes and their roles in memory and cognition.Research Topics
What are the circuit mechanisms of long-term memory?
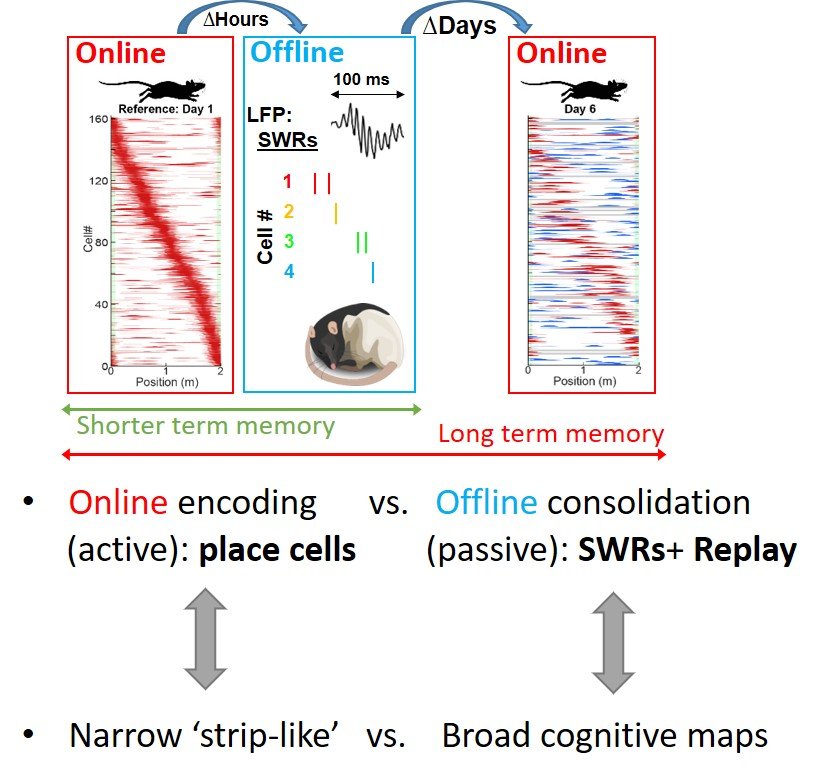
The overt learning that occurs while you are navigating the world around you is complemented by a covert form of re-learning, called memory consolidation, which occurs during your down-time. But how do these distinct memory mechanisms lead to distinct memory outcomes? A short thought experiment can help us tease apart these differences: imagine that you moved to a new city and go out to meet an old friend at a particularly great new bar. It takes you 20 minutes to get there, you then spend 3 enjoyable hours with him, and then another 20 minutes to get back. Now, due to both the length and quality of the time you spent there, it may seem logical for your brain to prioritize bar-specific memories for permanent consolidation. However, its only the 40 minutes you spent in transit which contain the information that you’ll need if you ever want to get back to that bar again in the future! Our recent work suggests memory consolidation plays a specific role in consolidating these less memorable, but often just as important aspects of experience. In other words, memory consolidation cognitively compliments ‘active’ learning by promoting the formation of ‘broad’ cognitive maps.
This division of cognitive labor in turns opens up exciting new questions. For instance, how does the local circuitry in the hippocampus, a structure associated with several forms of learning, change its function during active behaviors and during passive memory consolidation? And what can these differences teach us about the different cognitive ‘work’ carried out during each of these states? Our lab will answer these questions by combining cutting-edge two-photon imaging, electrophysiology, genetic targeting and closed-loop optogenetic manipulations together with advanced behavioral paradigms to dissect the initial formation and subsequent evolution of long-term memories.
How does neural diversity contribute to memory?
The hippocampal dentate gyrus is densely packed with sparsely active excitatory granule cells and previous work used to think about this circuit having only a single type of sparsely active place cells which encode the animal’s position within an environmental context by integrating across all the sensory information relevant to estimating its location. But by chronically imaging a large population of granule cells using two-photon Ca2+ imaging we have found an additional population encoding sensory cues. We will now figure out how different these cells are and why they are different. By using a combination of in vivo imaging, transgenic tagging and manipulation strategies as well as computational analysis of somatic and projection neural activity we will study the mechanisms by which multisensory information arriving from the external world generates the functional diversity in the DG representations.
Decoding of two-photon brain patterns during exploration. These same patterns spontaneously 'replay' during subsequent resting epochs.Untangling memory consolidation’s role in schizophrenia and aging
What are the circuit mechanisms of long-term memory deficits in mouse models for schizophrenia, and how can these deficits be rescued? While schizophrenia is most often associated with its positive, psychotic, symptoms and working-memory impairments, it is also associated with a range of cognitive deficits including large and well-documented impairments in long-term episodic memory. In a previous collaboration I showed that a mouse model for schizophrenia displayed not only impaired long-term spatial memory, but aberrantly high rates of sharp-wave ripples, which are short-lived, large population synchrony events strongly implicated in memory consolidation. Notably, alterations in sharp-wave rippling are also observed in age-related mild cognitive impairments as well as in Alzheimer’s disease – though the effect of these changes on their associated memory deficits remains little understood. By dissecting the different cognitive functions of memory formation and consolidation our lab’s work will aim to uncover new mechanisms by which pathologies affecting memory can come about – and in turn how specific interventions can ameliorate the resulting memory deficits.
What is the developmental basis to the brain’s response to stress and antidepressants?
Numerous studies have shown that the developmental time course of the formation of the hippocampal principal neurons matches their anatomical location and determines their wiring into the hippocampal circuits. Yet, how this developmental sequence affects the function of the neurons during cognitive behaviors remains unclear. In previous work, we have shown that adult born immature granule cells bidirectionally modulate diverse responses in the dentate gyrus; they increase remapping of place cells while decreasing remapping of cue cells. We will now ask whether such age-dependent specialization of function also applies to developmental neurogenesis or if the functional diversity emerges through experience after integration into the dentate network. We will also address clinically relevant questions of how do developmentally differentiated functional groups of dentate gyrus neurons respond to stress and antidepressant treatment and how can their activity be harnessed to develop novel circuit-based treatments of stress-induced psychopathologies, such as PTSD and depression.